Frequently Asked Questions

General
To reset your password, navigate to the PV247 login screen at www.pv247.com/login and follow these simple steps:
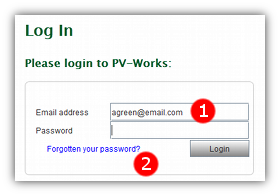
- 1 Enter the email address that is registered with PV247 in the ‘Email address’ field.
- 2 Click the ‘Forgotten your password?’ link.
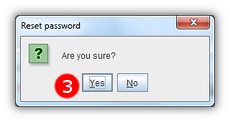
- 3 Confirm that you wish to reset your password
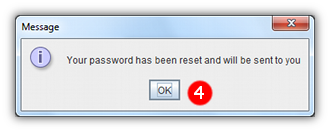
- 4 A message will appear, explaining that your new password will be sent to you by email.
- 5 When your new password arrives enter it into the login screen as normal.
If you have forgotten the memorable word that you use to log-in to PV-Works, please contact support by email at pv.support@ennov.com and we will reset it for you. Then you will be able to create a new one.
Please reach out to your sales consultant to confirm the transition to production. This will prevent your system from becoming disabled once it is 30 days old.
If you are a Premium package user, simply log-on to your separate production system using your production username.
If you are an Essentials user you should delete the test cases that you do not wish to keep and then reset the case numbers so that they can be re-used. To do this:
- Delete case by selecting the case, selecting “Delete case” from the “File” menu and follow the instructions.
- Repeat this for every test case to be deleted.
- Reset case numbers by selecting the “Reset Test Case Numbers” function from the “Configuration” button.
The prices displayed on this website are time-limited, these prices will remain in place until 31th December 2021. We intend to review prices once per year. However, for customers who wish to pay in euros or US Dollars, we reserve the right to adjust these prices if there are major currency exchange variations.
Data entry
To find out how to add contacts to a case, or correct errors with existing contacts please visit our Managing Contacts in PV-Works guide.
MedDRA Coding
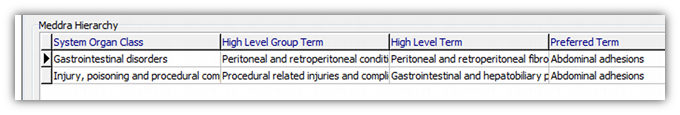
The Primary SOC is always the first one in the list shown when coding. It is also marked with a little black triangle on the left hand side.
New versions of MedDRA are issued on 1 March and 1 September each year and need to be in use on the first Monday in the month of May and November respectively. All updates will be performed on your behalf by the Ennov support team.
For all customers the update will take place over the week-end before the implementation date.
Note: all customers need to maintain a subscription for the MedDRA dictionary at all times.
Reported Address (CIOMS / MedWatch)
- Safety Reporting Group (ie the CRO)
- Market Authorisation Holder
- Manufacturer
- Sponsor
- 1. Enter the name and address using the Configuration | Contacts and Locations screen. Select the address type in the “Location type” box:
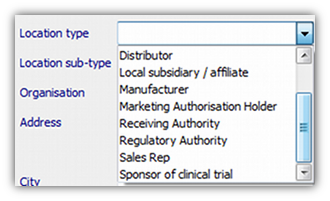
- 2. For Clinical Trials, define the trial itself (including its sponsor) using the Configuration | Clinical Trials screen
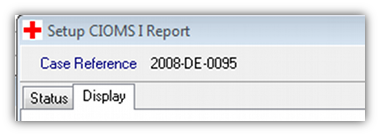
- 3. Select the appropriate address type when running the report. This is selected in the report set up screen for the CIOMS (or MedWatch) report. Go to the “Display” tab…

Reporting
When a PSUR is run PV-Works applied a number of conditions to determine the cases that will be listed. If you have a case that is not included but you believe it should then check the following conditions in the case.
When looking at a case that has been report approved remember that the highest version of the case is used. It is possible that the critical data item in the list below has been fixed in the master version but that the case has not been re-approved since the edit…. And the version is wrong.
Detailed Criteria:
For a case to be selected in a PSUR run it must satisfy ALL of the following rules. Failure to meet one of these conditions will result in the case being excluded:
- Case must be received between the two dates specified on the selection screen. The ‘date first received’ and the ‘date of latest follow up’ are both used for this purpose (ie include reports received initially or as follow ups during the target period). The two end dates are included in the filter.
- The case must be confirmed (on Case Identification screen)
- The case must include a company product that is one of the selected products for the run. In the current version the product will be included regardless of its role in the case.
- Case must be received:
| Source | Determined from |
|---|---|
| spontaneously | case identification screen – case type |
| from a clinical trial | case identification screen – case type |
| from a post-marketing study | case identification screen – case type |
| from the literature | case identification screen – source |
| directly from a regulatory authority | case identification screen – source |
- Case must be assessed for seriousness: Seriousness is determined using the following hierarchy, searching the case at each level until a non-null value is found. Only if all levels are exhausted without a seriousness value being found, will a selected case be determined to have no assessment (and is therefore excluded from the run):
- Seriousness of the most important event
- Case level assessment
- Case must be assessed for expectedness: Expectedness is determined using the following hierarchy, searching the case at each level until a non-null value is found. Only if all levels are exhausted without an expectedness value being found, will a selected case be determined to have no assessment (and is therefore excluded from the run):
- label specified by user on the PSUR set up screen
- CORE label (company [global] label)
- INV label (investigator’s)
- local label in country of occurrence
- case level assessment
In order to populate your XML Export with the correct sender details, it is important that you configure at least one Receiving Authority. This can be done from the Contacts and Locations option on the Configuration menu.
Click Add to create a new entry and set the Location type to ‘Receiving Authority’. Make sure you include full location and contact details, including an appropriate XML Sender:
Case management
If you have deleted a case (using the File | Delete case function) and need to have it restored, please contact support by email at pv.support@ennov.com and we will do this for you.
(Note that in a future release we will introduce a function for you to do this yourself).
If you have forgotten the memorable word that you use to log-in to PV-Works, you can reset this via the launch client application, or you can contact the support team (via the Ennov Support Portal) and we will reset it for you. Then you will be able to create a new one.
(Note that in a future release we will introduce a function for you to do this yourself).

