Why choose Ennov?
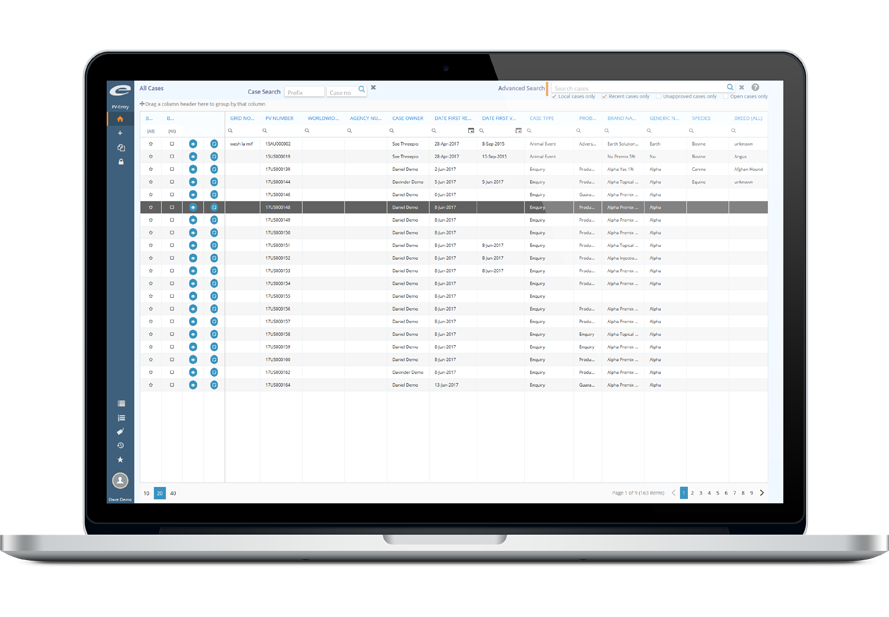
Our suite of applications covers a wide range of regulatory requirements

From leading pharmaceutical companies to emerging biotechs, we proudly serve over 450 Life Science companies and 500,000 users around the world.
We manage life science regulatory content
We work closely with regulatory authorities. Our regulatory intelligence and oversight enable us to keep our solutions always up to date with the latest regulatory requirements.

Consultants who understand your business
Our professional services team has extensive experience and expertise in the life science industry and will help you configure your solution to best suit your requirements.
More than 98% of our projects are delivered on time and within the initial budget.
To ensure the success of your projects, Ennov offers comprehensive training. We also offer to follow you during your first submissions.
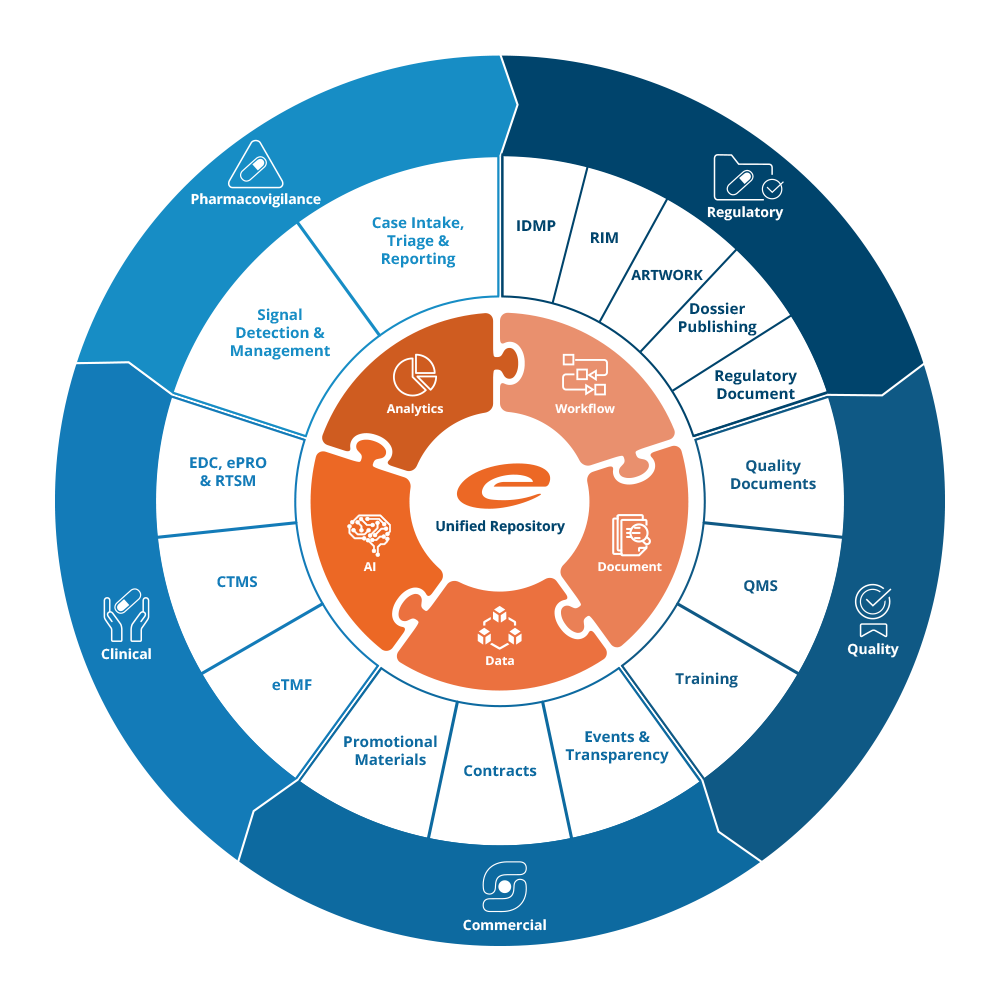
Configurable solutions for a low risk and total cost of ownership
Our solutions are designed to allow configuration by domain experts, with no IT skills required. This helps control the risk and cost of implementing projects while ensuring the scalability of implemented solutions.
This approach helps our customers deploy our solutions for a very competitive TCO.

Flexible and modern technological platform
We provide cutting edge web technology for our users:
- Web interfaces for universal access
- Scalable, fast and responsive
- Mature, robust technology with hundreds of thousands of satisfied users worldwide
Global presence
USA
Raleigh, NC
414 Fayetteville St.
Raleigh, NC 27601, USA
Phone: +1 (833) 366-6887
St Joseph, MO
520 Francis St,
St Joseph, MO 64501, USA
Europe
France
149 avenue de France
75013 Paris, France
Phone: +33 (0)1 40 38 81 38
UK
5 Eaton Court Road, Colmworth Business Park
Eaton Socon, St Neots Cambridgeshire PE19 8ER, UK
Phone: + 44 1 480 21 22 23
Belgium
Eedverbondkaai 242/003
B-9000 Gent
Belgium
+32 9 4961264
Asia
Vietnam
Sky City Tower, 6th floor
88 Lang Ha, Dong DaHanoi-Vietnam
India
Ennov Life Science Software Private Limited
1,2,3,4 Floor Krithika, Layout Plot No 3
Madhapur, Hyderabad-500081
Telangana, India
