Pharmacovigilance Workflow
This process must be controlled if temporal and quality goals are to be met. It needs to take into account the variety and quantity of cases handled, the need to meet deadlines derived from business and regulatory sources and the number of ‘hands’ through which a case passes from receipt to closure. Process management is an integral part of a product safety system and one that must be incorporated from the outset.
PV247 supports the pharmacovigilance process, with a level of support varying with the options selected:
View based process management
All offers include views that enable to track cases and facilitate process management:
- Cases that need to be coded
- Cases that need to be assessed
- Cases ready for reporting
- Cases that have been reported
- Cases where follow up has been received but not reported
- "To do” list
These views are clickable to perform corresponding actions. For example, in the view of “cases to be coded”, the user may double-click each row to execute the coding step and so remove the case from the worklist.
Reports are also included that list “cases due for submission” and the performance of reporting to a selected regulatory authority showing the percentage of cases reported on time.
Enterprise offer customers can extend this by creating their own views and reports to meet their process needs.
- Start date
- Target completion date
- Unlimited number of tasks
- Assignment of tasks to users (including yourself as reminders) or group of users
- No limitation as to the point in the process or the time where they are created
- Task description
- Notification of the task recipient that the task has been created
- Task highlighting
- Execution of PV247 functions for the selected task
- Tasks are color-coded to highlight their timeliness
Task oriented process management enables (in addition to view based process management) :
- To keep the task on a to do list until it is completed
- The task initiator to see if it was completed and when
- The task and its execution to become part of the case record and to be reviewable at any time
- Flexibility to handle any process / case situation, due to the tasks being defined individually
Workflow Based Process Management
Full-blown workflow module tailored to pharmacovigilance needs.
Each customer defines their own business processes graphically as a series of workflows (process maps) that include rules for branching, task assignment, expected duration and escalation. Different workflows can be created for different case types or user groups.
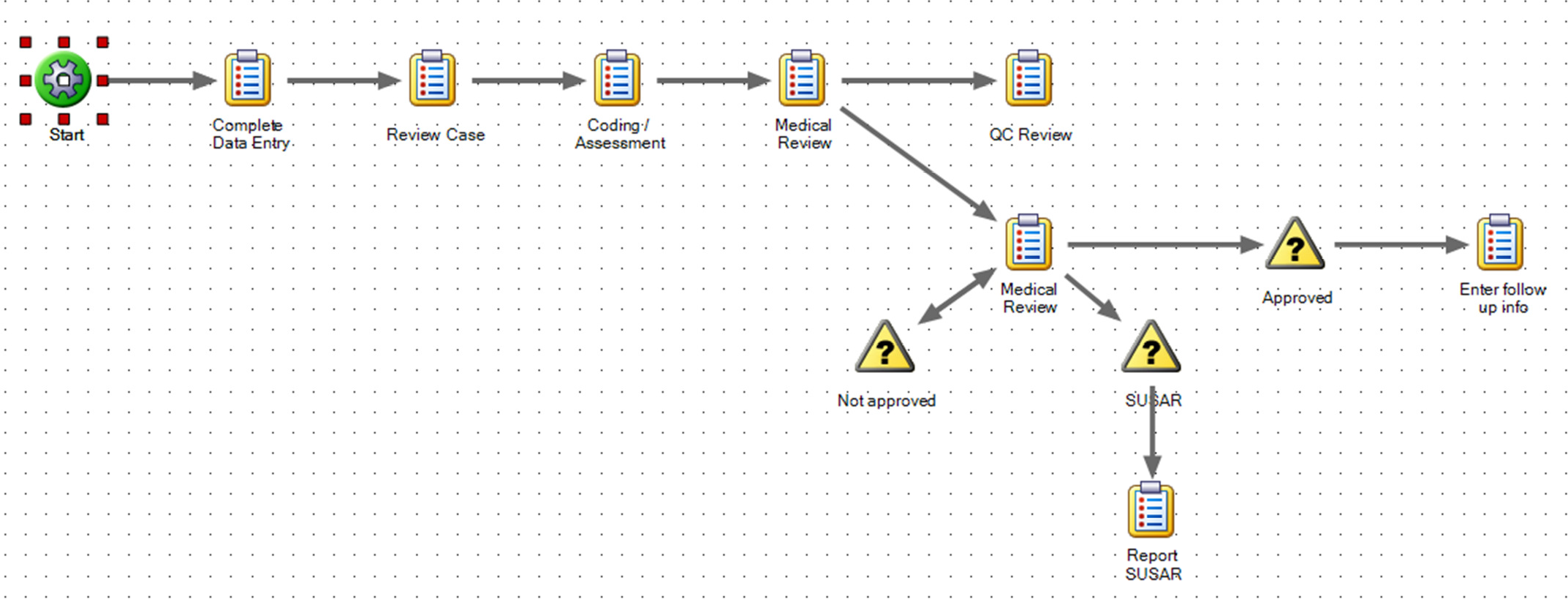
- ‘Active’ tasks are automatically included in their respective users’ ‘to do’ list (drawn from all active processes).
- Outstanding tasks are colour coded to highlight those that have priority or are overdue.
- Users can click on any task in the ‘to do’ list and are taken to the appropriate part of the system with the relevant data loaded so that the action step may be carried out (‘to do’ list fully integrated with the safety functions of the system).
- Upon completion of a task the workflow determines which tasks should be made ‘active’.
- Users are notified about these new active tasks so that they are ready for action.
- Supervisors can monitor the workload of a team or individual, and re-assign actions to other staff to overcome bottlenecks
Compliance with SOPs is achieved as the process map is followed, and the process ensures that all necessary tasks are completed for an individual case. Workflow driven process management is used by customers with large case volumes to ensure that every case is processed in accordance with its SOPs. Its value is increased for companies where staff from multiple locations are involved in the process.
Time targets are closely monitored and the system informs the user of the degree of any slippage, for instance relating to reporting deadlines.
Metrics are provided which can be used to track performance for individual tasks, workgroups or individuals. All data describing how each case progressed and how each action step was performed are stored.
Workflow driven process management can be used to handle the standard process, and ad hoc workflow tasks used together to manage unusual or one-off situations.
Ennov can help you design the first process map. This offer includes a review of your business process, design and testing of the process and its implementation.
Workflow driven process management enables to:
- Allow supervisors to review the workload of their team and re-balance it if necessary
- Ensure timely reporting, by linking tasks together and assigning “ideal execution time” to each
- Link tasks together so that when a task completes the system evaluates which task or tasks need to be created and then sets these running automatically
- Apply different rules to different cases, thanks to a comprehensive process design and decision points
- Create multiple process maps
- Activate tasks in parallel and assign them to the same person if appropriate.
- Create loops – when a critical data element is not entered the process can return to an earlier task to ensure completion
PV247 Workflows
- A pre-configured workflow implemented using the driven workflow process management described above.
- Three views that show cases that are currently in Data Entry, awaiting coding and assessment, or awaiting reporting
Timelines are set so that 7 and 15 day reports are submitted on time – if the user meets the targets set by the workflow.
The user is responsible for moving a case from one stage to another. The simplest way to achieve this is when a case is saved as the system will prompt with all open workflow tasks.
The workflow implemented by default for PV247 is deliberately simple, but helps manage the flow of reports and can be supplemented with ad hoc tasks as described above.

